A poster from the Clinical Research Support Center (CRSC) featuring new research on a sampling of IRB stipulations earned a Special Recognition Award from the Society of Clinical Research Associates (SOCRA) at its recent conference.
The poster analyzed four years of stipulation letters to gather data that will ultimately be used to improve IRB submissions and the research process. It was recognized as the best poster in the Clinical Research Management category.
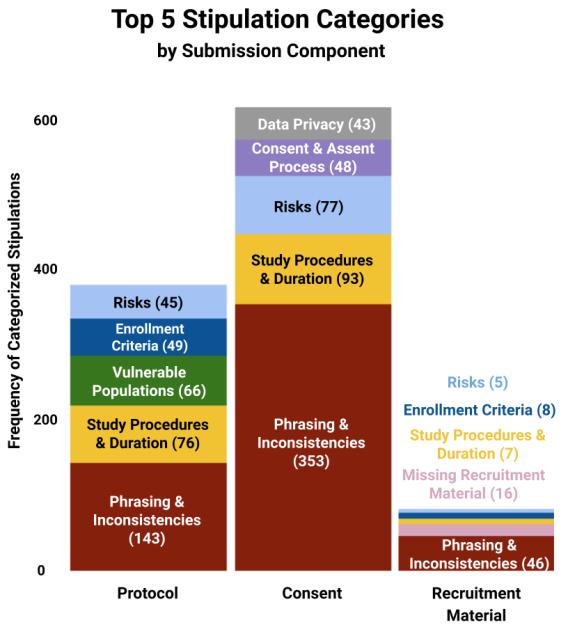
Conducted by CTSI faculty and staff who are involved in the CRSC, the research found the greatest number of IRB stipulations involved “phrasing and inconsistencies” within the submissions. The authors suggest a final documentation check could improve submissions and expedite the review process.
Driving research improvements
“CTSI’s Research Prep Group is always on the lookout for process improvement opportunities,” says Katherine Ingram, PhD, CCRP, who served as first author of the research. “This poster was a perfect example of how we start by collecting data, then use the results to guide our next steps. In this case, we were curious about what we could learn from stipulation letters, such as if we could find patterns that repeated.”
As the group pored over stipulation letters, themes emerged. The researchers classified each stipulation into 1 of 14 categories. Following are the five most common categories and examples for each:
- Phrasing and inconsistencies — Content must be consistent across all study materials, and participant-facing materials must be written at an appropriate reading level.
- Study procedures and duration — All study procedures must be described completely, including how they differ from standard of care.
- Risks — All risks need to be described in lay terms and avoid coercive language.
- Vulnerable populations — Rationale for inclusion and proper safeguards for vulnerable populations must be described, including a plan for assessing capacity to consent.
- Enrollment criteria — Study materials need to detail all inclusion/exclusion criteria and use clear, non-stigmatizing terminology.
Broadening impact
“There's not a lot of published academic work on common IRB stipulations, so we think what we found will be interesting to both the University of Minnesota researchers and those from other institutions around the country,” says Ingram.
The next phase will be to convene stakeholders to discuss the results, Ingram says:
“To improve the quality of their research protocols, we hope to help identify even small changes to reduce the number of stipulations that investigators and research teams receive.”