Find & Access Data

Access clinical, genomics, and biospecimen data for your research.
CTSI’s Best Practices Integrated Informatics Core (BPIC) gives University of Minnesota researchers access to high-quality data for their research.
How data can be used
- Study feasibility
- Retrospective and observational studies
- Recruitment
- Operational or quality improvement projects (non-research)
- Data feeds
- Education and fundraising
Request data
Office hours
Tuesdays 1:00-3:00pm
Thursdays 1:00-3:00pm (by appointment)
Office hours are held virtually, and can be accessed by contacting the Clinical Research Support Center (CRSC):
[email protected]
612-625-4000
Data sources and networks
Data sources
Data sources
Academic Health Center Information Exchange (AHC-IE)
Clinical Data Repository (CDR)
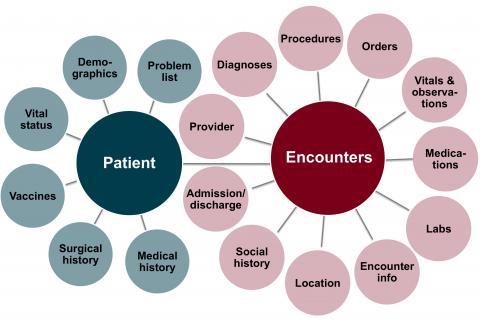
EHRs of more than 4.5 million patients seen at 8 hospitals and more than 40 clinics. Data is available for hospital visits starting in 2011; for Fairview Health Services clinic visits from 2005; and for University of Minnesota Physicians clinic visits starting from 2011. All start dates are approximate and depend on the date for the adoption of Epic® at each particular site.
Genomics data
Tumor (Somatic) Data and Germline Data
Fairview Tumor Registry
Minnesota Cancer Surveillance System (NAACCR)
CVIS
Discrete clinical data from Fairview Cath Labs
Optima/PaceArt
Cardiac medical devices interrogation data
Xcelera/Echo
Discrete data from the cardiology image management system
UMP Flowcast
Professional billing data (e.g., diagnoses, procedures, departments) for UMP providers
COVID-19 Registry
De-identified electronic medical records of COVID-19 patients as well as those with related conditions and symptoms. COVID-19 registry inclusion criteria.
Masonic Cancer Center Data Registries and Repositories
- Bone Marrow Transplant Research database (BMT): Captures post-transplant outcomes for BMT patients
- Laboratory information management system (LIMS): Subjects’ samples data (number of aliquots, analyte, etc.)
- FreezerWorks: Research samples inventory (freezer name, shelf, rows, box etc.)
BigMouth Network
Oral health records of more than 4.3 million consenting patients from institutions around the country.
OnCore® enterprise CTMS
Clinical Trial Data
Dental Records
AxiUm dental records from the University of Minnesota School of Dentistry
Minnesota Death Index
Minnesota Department of Health death records issued from 2011 to present for deceased individuals who were born in Minnesota, have died in Minnesota, or have ever had a permanent address in the state.
IBM MarketScan®
The University of Minnesota has obtained an institutional license to access IBM MarketScan® Commercial Claims and Encounters and Medicare Supplemental databases for years 2013 to 2021. The current license enables access to these data for approved researchers until May 2025.
MarketScan data provide claims (medical, prescription drug purchases, physician encounters, and hospital stays for the population as well as insurance enrollment) for a large population of commercially insured patients and Medicare beneficiaries with supplemental health coverage.
For more details about access to this data, fees, and restrictions, please refer to the Data Use Agreement Document.
Spatial Data:
- Patient records are geocoded and enhanced with latitude / longitude / block data elements
- Census data at the block group level from the 2010 Census.
- The Clinical EMR data is geocoded and enhanced latitude / longitude / block data elements
UMLS Data
Standard Terminology and code sets including ICD-9/10, LOINC, NDF-RT, RxNorm, CPT, HCPCS
Other
Datasets obtained by Researchers (e.g., OptumLabs, AHRQ/HCUP, CMS, VA, etc.)
Data sharing networks
Data sharing networks
The CTSI Informatics core participates in several data sharing networks, the goal of which is to facilitate and streamline multi-site clinical research.
Submit a BPIC consultation request to learn how data sharing networks can help your research.
Accrual to Clinical Trials (ACT)
- Supporting cohort identification across 21 sites (approximately 40M patients).
- Contact us to query the NCATS-ACT Network.
All of Us Research Hub
- One of the largest, most diverse, and most broadly accessible datasets ever assembled.
- Explore aggregate data including genomic variants, survey responses, physical measurements, electronic health record information, and wearables data.
MARCH
- Offers investigators a streamlined infrastructure for undertaking studies that are better served at multiple sites.
National COVID Cohort Collaborative (N3C)
- One of the largest collections of secure and de-identified clinical data in the U.S. for COVID-19 research.
TriNetX
- Provides anonymized cohort search over UMN data to major Pharmas so that new trials are efficiently initiated.
More about the data request process
Who can request
Who can request clinical data?
User authorization
Each partner manages its own process for authorizing its users, but the requirements generally include that you are an employee in good standing, have completed HIPAA training, and have signed an attestation form in which you agree to terms for use of the data (e.g. you will only use the data for the purposes specified in the original request form). If you are affiliated with more than one of the partners, your authorization will be via the affiliation most relevant to your data request. It is not your responsibility to ensure your authorization before submitting a request; we will verify your authorization once your request has been submitted.
External collaborators
External collaborators cannot request data themselves, but qualified University-affiliated individuals or departments can be granted access in some cases. To do so, a University member must secure a sponsored guest x500 account for their external collaborator. This is a free service that can be requested via the Office of Information Technology.
More information for students and employees of UMN
Addressing the following requirements as soon as possible will allow us to deliver data to you more quickly:
HIPAA training
- The University's HIPAA Privacy and Data Security Training program consists of four separate online courses that are part of the "Public Jobs: Private Data" training program.
- If you've completed the training, you do not need to re-take it. A record of all training completed while at the University is available online through the Training History link at the MyU website. You also can contact the Health Information Privacy and Security Office at [email protected].
Attestation form
- You must provide attestation to indicate that you comply with the terms of AHC Information Exchange (IE) data use. To attest, complete this short online form, which will require that you sign in with your university ID (x500). Your attestation will be valid for one year.
IRB approval guidelines
Do I need IRB approval?
Research
IRB review and approval is required for research falling under the federal definition of human subjects research. IRB approval is required for data requests that require identifiable private information and involve a systematic investigation designed to contribute to generalizable knowledge.
Visit the U of M's IRB website for more guidance (e.g., Does My Research Need IRB Review?) and instructions for submitting a modification to a protocol or adding personnel.
Activities preparatory to research
Data requests to determine study feasibility or plans for participant recruitment do NOT require IRB review. Participant recruitment itself, however, does need IRB approval. For more information on the disclosure of PHI for activities preparatory to research, see the related Administrative Policy.
Non-research activities
If you need data in support of clinical operations or quality improvements, you do not need IRB approval however, approval by a Data Steward is required.
Recruiting participants
How can I use my data to recruit participants?
If you would like to send recruitment letters to the patients you have identified with the assistance of BPIC, we will work with you and Fairview Research Administration (FRA) to coordinate such a mailing.
- When you submit your data request to BPIC, let us know that you plan to send out recruitment letters via Fairview Research Administration.
- Fairview will send out recruitment letters on your behalf provided that your study will occur at Fairview or the University of Minnesota and that you have UMN IRB approval for your study. Read the Fairview Research Recruitment Mailing Guidelines.
- Contact FRA at [email protected] as early as possible to alert them to your plans for a recruitment mailing.
Policies and procedures
Policies and procedures
Support and technical assistance
Support
How do I get informatics support?
In addition to providing data, experts from BPIC also can help you work with that data. Contact us at [email protected] if you want to:
- Get help managing the data you received through our data request process.
- Get support for the informatics aspects of your grant applications.
- Learn about informatics experts beyond CTSI.
Consultations are available at no charge to researchers at the University of Minnesota, Fairview, and University of Minnesota Physicians. Request a consultation via our website or by submitting a request form directly.
Training
Contact [email protected] to set up a training session or to learn more about our services, data and tools.
Who do I contact if I need help?
General questions and assistance?
Please contact [email protected]
Forgot your password?
Password assistance for University of Minnesota students and staff, visit IT@UMN
x500:
Use your University of Minnesota x500 username and password to access: i2b2, SHRINE, Duo, Box and vpn
AD Account:
Access the Data Shelter via remote desktop using your AD username and password.
Rates
Initial consultations:
Free
Grant proposal preparation:
Free
Services:
$120/hour: Internal hourly rate
$95/hour: For Cancer Center members
Contact
Gretchen Sieger
Director of Operations, Clinical Informatics
[email protected]
Steve Johnson, PhD
Scientific Director, Clinical Informatics
[email protected]
Melissa Hansen
Research Navigator
[email protected]
612-625-CTSI (2874)