Minnesotans now have an easier way to find clinical trials and other health research studies at the University of Minnesota, thanks to enhancements to StudyFinder. CTSI updated the website so it features more research studies, has study landing pages, includes more information about each study, and offers a better search experience.
More studies to be featured
StudyFinder now includes data from the OnCore clinical trial management system, expanding the inventory of studies featured on the site.
This means the website now includes observational studies and survey-based research, in addition to the UMN studies it automatically identifies from ClinicalTrials.gov. Investigators can also choose to have a study individually added or removed as appropriate.
As of August 2021, there are more than 450 UMN research opportunities listed on StudyFinder.

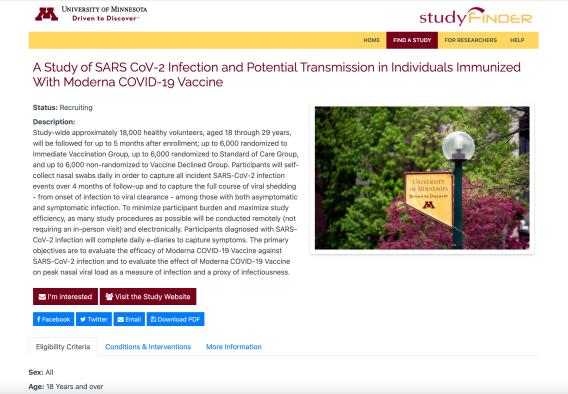
Study landing pages
StudyFinder now automatically generates a dedicated landing page for each study.
StudyFinder landing pages provide researchers a convenient place to direct people without the effort of manually creating and managing a separate webpage. Webpages can easily be shared on social media or via other channels to raise awareness and help study teams recruit participants.
On these landing pages, potential participants can find information about the study, learn who is eligible, what the intervention is, and how to contact the study team.
StudyFinder handles this by refreshing data nightly from:
- ClinicalTrials.gov
- OnCore
- ETHOS
Researchers can also request changes to the content shown on their landing pages, such as to add an IRB-approved photo or link to a study-specific website.
More information about each study
StudyFinder now includes a brief, IRB-approved description of each study as well as the name of the principal investigator (PI) and study contact. Information is pulled from ETHOS and OnCore, respectively.
Pulling contact details from OnCore helps increase the information’s accuracy and gives study teams the ability to quickly and easily make changes themselves.
Better search experience
StudyFinder’s search capabilities have been enhanced, making it easier for potential participants to find research opportunities by keyword, condition, researcher name, or health topic.
Plus, researchers have new options to make their studies more findable within StudyFinder. Through OnCore, study teams can select categories and provide keywords. This helps ensure their studies are easily found when people search in StudyFinder.
OnCore integration also allows studies to be filtered and displayed by location and by researcher, making it easier to share opportunities with patients and potential participants.

An open-source tool used by CTSA hubs nationwide
Ever since StudyFinder launched in 2013, it has been expanding nationally to other Clinical and Translational Science Award (CTSA) program hubs. The open-source tool is available to all hubs and in addition to the University of Minnesota, StudyFinder has been adopted by:
- Penn State University
- Virginia Commonwealth University
- University of Texas Southwestern
- University of Wisconsin (UW Health)
- Two other CTSA hubs collaborating on a statewide website
StudyFinder gives these institutions a simpler way to create a comprehensive, accurate inventory of their enrolling studies, while making it easy for their communities to find a study that’s right for them.
For questions about managing your study on StudyFinder check out the website’s For Researchers section or email the StudyFinder team at [email protected].
