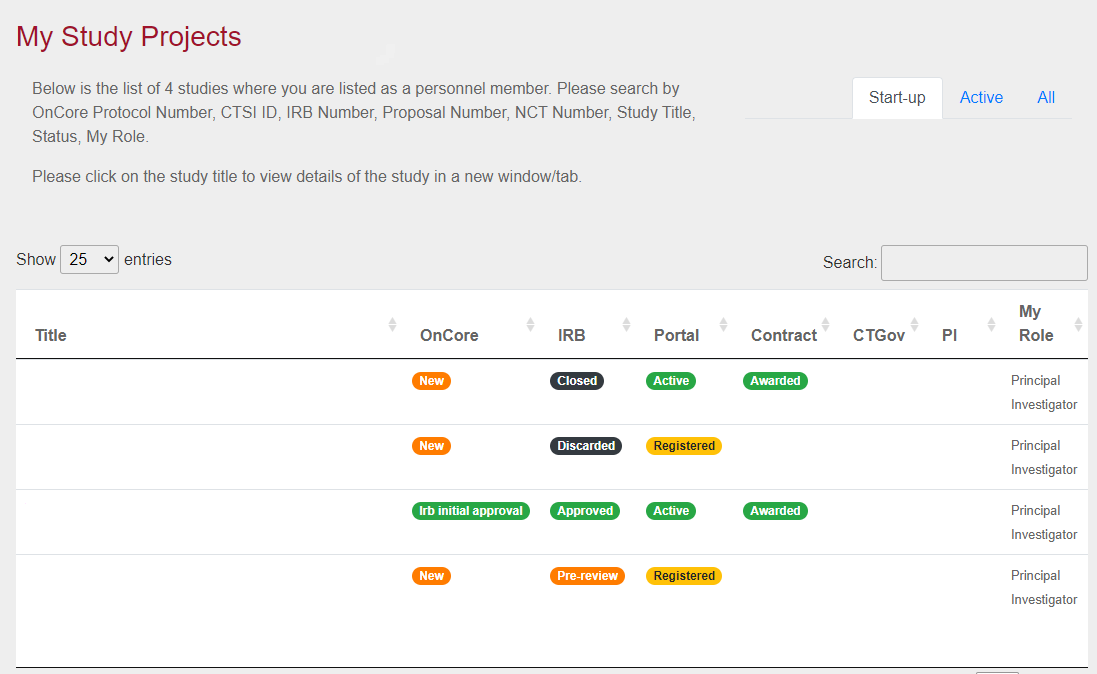
University of Minnesota researchers have a new tool for quickly and easily understanding their study’s status with the debut of the Clinical Research Project Status Tool.
One dashboard for multiple systems
The tool pulls study data from several systems used by researchers and plugs it into a single, user-friendly dashboard. Data sources include:
- OnCore Clinical Trial Management System (CTMS)
- ETHOS
- EFS
- Clinical Translational Research (CTR) Portal
- ClinicalTrials.gov
Know your project’s status and what to do next
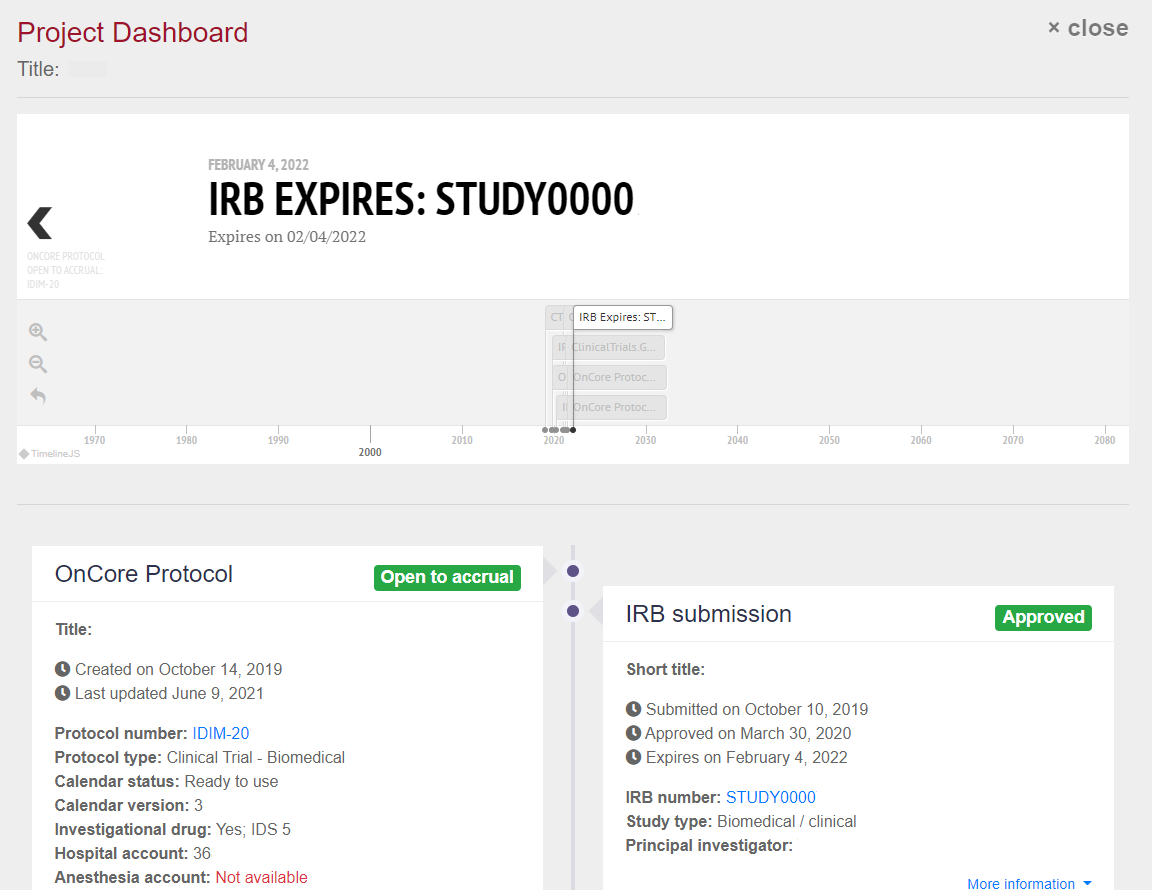
By displaying key data in one place, the tool gives researchers a fast, easy way to understand their study’s status and identify any issues.
Investigators and study teams can use the tool for a number of things, including to:
- Determine IRB submission status and expected expiration date.
- View sponsor contract status, including negotiation status for Business and Industry contracts.
- See OnCore protocol and calendar build progress.
- Identify which CTSI services have been requested and the corresponding status.
- Verify ClinicalTrials.gov information, including if there are any problems that need to be addressed, and review the last modified dates.
Stemming from researcher feedback
The idea originated from design sessions held by the Clinical Research Support Center in 2017.
Researchers and staff said they found it difficult to know the status of their studies. Traditionally, they’d need to check several systems to understand where each project was in the complex research process.
With this tool, information from several systems is consolidated into one place. And, much like an app that tracks the status of a pizza delivery, it provides an easier way to see the status of a study.
A collaborative effort
The Medical School developed the tool in collaboration with CTSI as well as the UMN Health Sciences Technology (HST) office, Institutional Review Board (IRB), and Sponsored Projects Administration. CTSI and the Medical School partnered to fund the project.
